Good manufacturing practices (GMP) are a collection of principles and standards that ensure products are consistently manufactured and controlled to fulfill quality requirements.
These practices are critical for organizations and industries, particularly those involved in food, pharmaceuticals, and healthcare items, because they help to minimize contamination, errors and maintain consumer safety.
In Singapore, where sectors are highly regulated and competitive, knowing and implementing GMP is critical for preserving compliance and obtaining local and international consumer trust.
This article will look at the concept and importance of GMP, as well as the major components that companies must consider, and how GMP is governed in Singapore. We will also discuss good manufacturing methods in the food and pharmaceutical industries.
By grasping these ideas, Singapore enterprises may enhance product quality, streamline processes, and meet regulatory requirements, thereby supporting development and strengthening their position in the global market. Here is the explanation of the GMP standard with a complete guide. Learn more here!
- Good manufacturing practices (GMP) are essential procedures that help businesses ensure consistent product quality and safety throughout the entire production process.
- 5 main components of GMP are people, product, processes, procedures, and premises
- Principles and aspects of GMP include hygiene, documentation, staff training, quality control, inspections, and equipment maintenance to maintain smooth operations, and others
- To comply with GMP standards guidelines are follow GMP standards, apply for certification, maintain records, and be inspected
- ScaleOcean ERP Manufacturing streamlines GMP compliance by integrating quality control, real-time monitoring, and automated documentation.

What is Good Manufacturing Practice (GMP)?
Good Manufacturing Practice (GMP) is a quality assurance system that guarantees products are consistently made to meet established quality standards, especially in manufacturing industries, such as health, pharmaceutical, and many others.
It includes stringent operational and quality controls across industries such as pharmaceuticals, food, and cosmetics, embedding safety and quality into manufacturing and supply processes. Regulatory bodies typically oversee compliance with GMP guidelines, which may lead to certification.
Why is Good Manufacturing Practice (GMP) important?
Following strong manufacturing processes allows businesses to avoid costly mistakes and product recalls. These methods ensure that products continuously meet quality and safety requirements, while also supporting the entire product lifecycle process to maintain consistent standards.
Most significantly, GMP safeguards consumers by avoiding potentially dangerous items from entering the market. This fosters trust between organizations and their customers, which is critical to long-term success.
In high-risk industries such as food and pharmaceuticals, omitting basic controls might lead to significant consequences. GMP operates as a safety net, protecting each batch of products. It ensures that products are reliable and safe before they reach consumers. Companies that adhere to GMPs reduce hazards and effectively protect public health.
5 Main Components of Good Manufacturing Practice
Good Manufacturing Practice (GMP) is a critical framework for ensuring that products are produced consistently and controlled to quality standards. It focuses on maintaining high levels of hygiene, safety, and efficiency throughout the production process.
Here are the five main components that contribute to successful GMP standards, ensuring safe, high-quality products.
1. People
People are one of the most crucial components of GMP. Proper training, skill development, and adherence to health and safety protocols are essential for employees involved in manufacturing.
GMP emphasizes the importance of having qualified personnel who understand the standards, practices, and regulations that govern the production process to ensure consistency and quality.
2. Product
The product component of GMP focuses on ensuring that the final product is manufactured according to predetermined specifications. Proper handling and storage of products throughout production are key to maintaining their integrity.
This involves maintaining high standards for raw materials, monitoring the production process, and ensuring that the final product meets safety, quality, and regulatory requirements, all while optimizing the cycle time process to improve efficiency and reduce delays.
3. Processes
Processes under GMP refer to the defined methods and systems used to manufacture products. These processes must be standardized and controlled to ensure reproducibility, consistency, and compliance with quality standards.
Each step in production, from raw material handling to packaging, must be carefully monitored and optimized to minimize errors and risks that could compromise product quality.
4. Procedures
Procedures are detailed instructions that guide every aspect of production, ensuring consistency and compliance with GMP standards. These include cleaning procedures, quality checks, equipment maintenance, and material handling, along with a good bill of materials to ensure accuracy and efficiency.
By following documented procedures of GMP standards, manufacturers can prevent deviations and ensure that the manufacturing process aligns with regulatory standards and best practices.
5. Premises
Premises refer to the physical environment in which manufacturing takes place. GMP requires that facilities be clean, well-organized, and designed to prevent contamination or errors.
This includes proper ventilation, lighting, sanitation, and maintenance of equipment and machinery. A well-maintained facility ensures that products are manufactured in a controlled and safe environment, minimizing the risk of contamination or defects.
12 Principles and Aspects of Good Manufacturing Practice (GMP)
Good manufacturing procedures include several key components that work together to assure product quality and safety. These components include everything from maintaining cleanliness to ensuring accurate documentation and comprehensive training.
Each principle is crucial to ensuring that the manufacturing process runs smoothly and meets regulatory requirements, while optimizing the production capacity to meet market demands. The following are the main principles that lay the groundwork for efficient GMP implementation:
1. Quality Assurance
Quality assurance is a fundamental aspect of GMP, ensuring products consistently meet predefined quality standards. It involves rigorous testing, monitoring, and validation throughout the manufacturing process, including at various Work In Progress (WIP) stages to ensure quality at every step.
By implementing quality assurance systems, manufacturers can detect and rectify any issues early, minimizing defects and ensuring the final product meets safety and efficacy requirements.
2. Industry-Wide Application
GMP is applied across various industries, including pharmaceuticals, food and beverages, cosmetics, and medical devices. Its universal application ensures that manufacturers in different sectors adhere to the same rigorous standards, promoting safety and quality.
Industry-wide adoption of GMP helps maintain consumer trust and ensures that products are safe for use, regardless of the sector.
3. Comprehensive System
GMP is a comprehensive system that covers every aspect of manufacturing, from raw material procurement to final product delivery. It includes standardized procedures for equipment calibration, employee training, facility maintenance, and environmental controls.
By establishing a detailed and all-encompassing system, GMP standards ensure that every stage of production contributes to product quality and regulatory compliance. Implementing takt time in the system further optimizes production efficiency while maintaining quality and compliance.
4. Regulatory Oversight
Regulatory oversight is a key component of GMP, ensuring that manufacturing processes align with local and international regulations. Authorities like the FDA or EMA conduct inspections and audits to verify compliance.
This oversight helps to prevent unsafe or substandard products from reaching the market, protecting public health and ensuring that manufacturers adhere to required quality standards.
5. Certification
Certification is a critical aspect of GMP, validating that a manufacturer complies with all required practices and standards. Obtaining GMP certification involves thorough audits and assessments by regulatory bodies or third-party certifiers.
This certification assures consumers and stakeholders that the manufacturer is committed to producing high-quality, safe products, adhering to global standards.
6. Hygiene and Cleanliness
To avoid contamination, factories and equipment must be kept immaculate at all times. Clean environments lower the risk of defects and safeguard customer health. Proper sanitation measures should be enforced on a regular basis. Maintaining hygiene is the first line of defense in quality control.
7. Proper Documentation
Every stage of production must be meticulously documented to maintain traceability. Accurate documentation facilitates the identification of concerns and aids audits. It also demonstrates that procedures were followed correctly. This transparency is necessary for regulatory compliance.
8. Staff Training
Employees must be well-trained on GMP standards and procedures. Proper training ensures that employees understand their obligations clearly. It reduces errors caused by misunderstandings or neglect.
Ongoing education keeps skills and knowledge up to date. Staff who have been properly taught play an important role in ensuring consistent product quality.
9. Quality Control
Regular quality checks during the manufacturing process ensure product uniformity. These controls identify variations early on, preventing faulty products from reaching consumers. Quality assurance contributes to customer safety and satisfaction.
It also helps to maintain the company’s reputation. Implementing good quality control lowers waste and operational costs, improving overall production efficiency.
10. Frequent Inspections
Routine audits and inspections detect possible issues before they worsen. These inspections ensure compliance with GMP rules and operational efficiency. Early discovery enables timely corrective actions.
Inspections ensure that manufacturing improves on a constant basis. They also instill confidence in regulatory agencies and customers.
11. Equipment Maintenance
Machinery should be serviced on a regular basis to ensure proper and safe operation. Equipment that is well-maintained is less likely to break down and cause contamination.
Scheduled maintenance improves operating reliability. This promotes consistency in product quality. Preventive maintenance also increases the life of expensive equipment.
12. Material Management
Ensuring that raw materials and components are sourced, stored, and handled correctly to prevent contamination and deterioration is crucial, especially in a make-to-order approach. Materials should be checked for quality before use in production to maintain standards.
Good Manufacturing Practice (GMP) Regulation in Singapore
Good manufacturing practice in Singapore’s regulations mixes international and local standards to assure quality. The Health Sciences Authority (HSA) is in charge of monitoring and implementing these GMP regulations.
To operate legally in Singapore, local manufacturers must follow certain rules, which include thorough manufacturing process planning to ensure compliance and product consistency. This control contributes to high levels of safety and quality in production operations.
Singapore’s GMP norms closely adhere to the World Health Organization (WHO) standards and other global benchmarks. According to Pacific Bridge Medical, starting October 1, 2024, drug companies submitting NDAs, GDAs, or minor variation applications in Singapore must provide evidence of GMP compliance for their drug substances.
Aligning with international practices enables Singaporean businesses to compete globally. It also simplifies the export process for Singapore-made products. This relationship helps local firms thrive and access new markets.
Good Manufacturing Practice (GMP) Standards
Good Manufacturing Practice (GMP) standards are essential guidelines that ensure the safety, quality, and consistency of products in industries like pharmaceuticals, food, and cosmetics. These standards are closely tied to manufacturing process planning steps to optimize production and ensure compliance.
These standards help maintain regulatory compliance and safeguard consumer health by focusing on key aspects such as quality, validation, and audits. Below are the crucial components of GMP standards.
1. Quality Team
A dedicated quality team is integral to the GMP process. This team is responsible for overseeing all manufacturing activities, ensuring that products are consistently produced according to established quality standards.
The quality team monitors every phase, from raw material inspection to final product testing, ensuring all processes comply with GMP regulations. This includes planning for material requirements to guarantee the safety and efficacy of the products.
2. Validation and Confirmation
Validation and confirmation are critical in GMP to ensure that all processes, systems, and equipment consistently produce products that meet quality standards.
This involves testing, documenting, and verifying that manufacturing processes and equipment perform as expected under specific conditions. Validation confirms that every step in production aligns with GMP guidelines and produces consistent, high-quality results.
3. Training of GMP Guidelines
Training is an essential component of GMP standards to ensure all personnel understand and adhere to manufacturing best practices. Regular training programs are required for all employees, from management to workers on the production floor.
This ensures they are familiar with GMP guidelines, operational procedures, and quality control measures to minimize errors and maintain consistent product quality.
4. Create GMP Audit Checklist
Creating a GMP audit checklist is crucial for internal assessments and ensuring continuous compliance. This checklist includes a comprehensive list of GMP standards and requirements for the manufacturing process.
It serves as a guide during audits to evaluate whether all necessary protocols are followed, identify gaps in compliance, and ensure corrective actions are implemented to maintain quality standards.
5. Audits of GMP
Audits are an essential part of maintaining GMP compliance. Regular audits, both internal and external, are conducted to assess adherence to GMP standards.
Auditors evaluate processes, procedures, and documentation, identifying areas of improvement and ensuring that corrective actions are taken. These audits help prevent regulatory issues, identify potential risks, and ensure the continuous safety and quality of products.
How to Comply with GMP Guidelines
Complying with Good Manufacturing Practice (GMP) guidelines is essential for ensuring the safety, quality, and consistency of products. Adherence to GMP not only meets regulatory requirements but also builds consumer trust.
To ensure compliance, manufacturers must follow key steps, including following GMP standards, applying for certification, maintaining records, and undergoing inspections.
1. Follow GMP Standards
The first step in GMP compliance is to understand and implement the necessary standards. This involves adhering to documented procedures for quality control, sanitation, equipment maintenance, and personnel training.
By establishing a GMP-compliant environment, companies ensure that all manufacturing processes are performed to the highest standards, preventing errors and ensuring product consistency and safety.
2. Apply for Certification
Obtaining good manufacturing practices certification is a formal process that demonstrates a company’s commitment to meeting regulatory and industry standards.
Manufacturers must apply to regulatory bodies or third-party organizations, undergo thorough audits, and prove they comply with GMP guidelines. Certification provides credibility and reassures consumers and stakeholders that products meet safety, quality, and regulatory requirements.
3. Maintain Records
Maintaining accurate and comprehensive records is crucial for GMP compliance. Manufacturers must document every step of the production process, including raw material sourcing, equipment calibration, production schedules, and quality control checks.
Proper record-keeping ensures traceability, supports transparency, and provides evidence of compliance during audits or inspections, helping to maintain product quality and safety.
4. Be Inspected
Regular inspections by regulatory bodies are essential for ensuring ongoing GMP compliance. These inspections assess if manufacturing practices align with GMP guidelines, checking for issues such as equipment malfunctions, contamination risks, or documentation errors.
Companies must be prepared for scheduled or surprise inspections to maintain certification and demonstrate their commitment to continuous improvement in quality and safety standards.
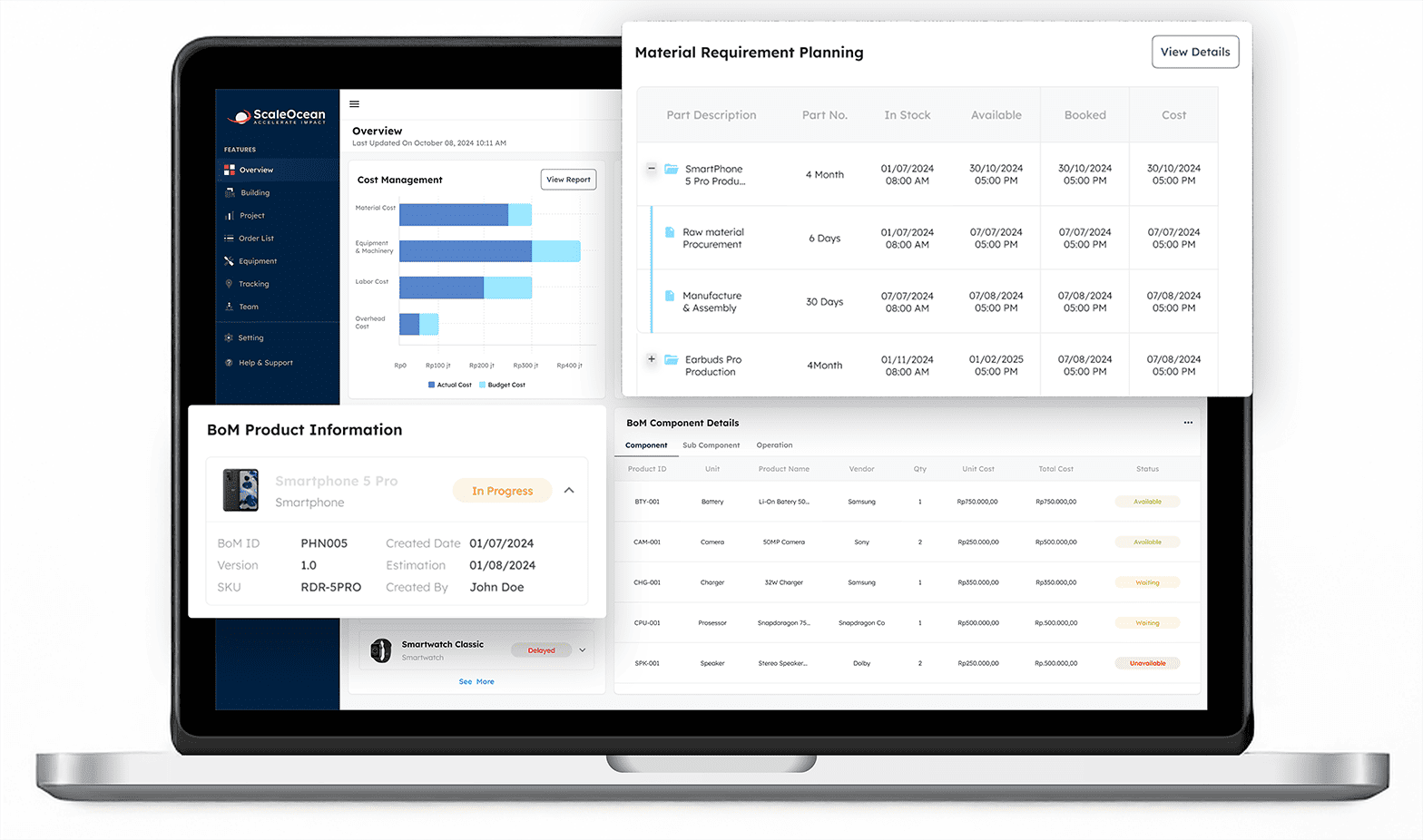
You can use ScaleOcean ERP manufacturing to comply with GMP (Good Manufacturing Practice) guidelines through a series of integrated features that ensure regulatory compliance, quality control, and traceability throughout the manufacturing process.
Take a free demo now to get this solution for your business.

Examples of Good Manufacturing Practices Industries
Good Manufacturing Practices (GMP) are essential across various industries to ensure product safety, quality, and regulatory compliance. Different sectors rely on GMP to maintain high standards in their production processes.
By adhering to GMP guidelines, these industries can minimize risks, enhance product consistency, and protect consumer health. Here are examples of how GMP is applied across key industries.
1. GMP for the Food Industry
In the food industry, good production techniques are critical to preventing contamination. Companies must maintain careful control over the quality of raw materials utilized in production.
To avoid product spoiling, production areas must be kept clean. Staff hygiene is also closely maintained to limit the likelihood of foodborne illness. Together, these methods make food production safer for consumers.
Regular inspections and food safety audits enable businesses to continually maintain high standards. Process improvements are performed based on audit results to address any issues discovered.
Furthermore, firms prioritize good packing to prevent items from damage and infection. Safe storage and dependable transportation guarantee that food remains fresh until it reaches the consumer’s shelf.
2. GMP for Pharmaceutical Industries
When it comes to medicine, the risks are really great. Good pharmaceutical manufacturing processes ensure that drugs are safe and effective. They ensure that medicines are pure and free of dangerous ingredients.
Current good manufacturing standards play a vital role in detecting and preventing errors early. According to HSA Singapore, the revised Annex 1 to the PIC/S GMP Guide for sterile product manufacturing will take effect on 25 August 2023, setting updated standards for sterile medicine production, except for Section 8.
These practices include numerous crucial actions, such as equipment sterilization to prevent infection. To assure quality, each medicine batch is closely monitored for consistency.
Accurate and detailed record keeping is required for traceability and accountability. Pharmaceutical businesses must strictly adhere to these guidelines to preserve patient safety and meet legal requirements.
3. Food and Beverages
In the food and beverage industry, GMP ensures the safe handling, processing, and packaging of food products. This includes controlling the hygiene of facilities, preventing cross-contamination, and ensuring proper storage of ingredients.
Regular sanitation, pest control, and monitoring of production environments help maintain the quality and safety of consumables, protecting public health.
4. Cosmetics
Cosmetics manufacturers adhere to GMP to ensure the safety and effectiveness of beauty products. This includes controlling ingredient quality, using sanitized equipment, and maintaining precise formulations to avoid contamination.
GMP also dictates that cosmetic products undergo thorough testing for skin safety, packaging integrity, and product stability to meet regulatory standards and consumer safety requirements.
5. Medical Devices
In the medical device industry, GMP plays a critical role in ensuring that devices are produced with consistency and meet regulatory standards. This involves controlling the manufacturing process, including the sterilization of components and materials, precise labeling, and thorough testing for safety and efficacy.
GMP also includes documentation and traceability to track devices and ensure compliance with safety protocols.
Streamline GMP with ScaleOcean Manufacturing Software
ScaleOcean Manufacturing ERP is a robust software solution that assists manufacturers in optimizing their operations while adhering to good manufacturing standards (GMP).
ScaleOcean combines quality control, real-time monitoring, and automated documentation to eliminate errors and increase efficiency. ScaleOcean enables businesses to maintain consistent product quality, ensure regulatory compliance, and improve overall operational transparency.
We provide a free demo so you can experience firsthand how it improves your organization. Furthermore, with the CTC grant help available, implementing this ERP solution becomes even more feasible for Singapore-based enterprises. The following is a list of main features from the ScaleOcean software:
- Comprehensive Quality Control Integration: Built-in quality control modules ensure every batch passes strict inspections, maintaining GMP-compliant product quality.
- Real-Time Production Monitoring and Traceability: Offers live tracking of all processes, from raw materials to finished goods, ensuring full traceability and accurate records.
- Automated Documentation and Audit Readiness: Automatically records production steps and quality checks for clear, error-free documentation, simplifying audits and compliance.
- Preventive Maintenance and Equipment Management: Schedules maintenance to reduce downtime and contamination risks, supporting clean and well-maintained GMP environments.
- Employee Training and Compliance Tracking: Manages training programs and tracks GMP compliance, keeping staff informed on crucial quality and safety standards.
Conclusion
Implementing good manufacturing practices is critical for guaranteeing product quality, safety, and regulatory compliance in any manufacturing operation. Companies that adhere to GMP requirements and use technology can reduce risks, enhance productivity, and foster more consumer trust.
The integration of quality control, real-time monitoring, and correct documentation is critical for maintaining these standards. To assist firms in meeting these objectives, the ScaleOcean manufacturing ERP system is designed to provide a full solution to streamline GMP operations.
ScaleOcean, which includes automated quality checks, manufacturing traceability, and compliance tracking, helps firms follow requirements while improving operational performance. Take a free demo now to get the best solution for your business.
FAQ:
1. What are the basics of GMP?
Good Manufacturing Practice (GMP) is a system aimed at ensuring products are consistently manufactured and controlled in line with predefined quality standards. It is designed to reduce risks associated with pharmaceutical production.
2. Is GMP mandatory?
Legal requirements: In addition to adhering to government regulations, certain businesses in India must obtain GMP certification by law. For instance, the pharmaceutical industry is heavily regulated, and companies involved in drug production are legally obligated to acquire a GMP certificate.
3. Is GMP the same as ISO?
GMP requires detailed documentation of each production step, with strict record-keeping and traceability standards. In contrast, while ISO also mandates documentation, its approach is more flexible, emphasizing process improvement rather than rigid regulatory compliance.
4. What happens if GMP is not followed?
Failure to comply with GMP can lead to various consequences, including:
Warning Letters: Issued by regulatory bodies like the FDA or EMA when major GMP violations are found during inspections.
Import Alerts: Products may be prohibited from entering certain markets.
Product Recalls: Companies may be compelled to recall non-compliant products from the market.








 PTE LTD..png)
.png)

.png)






.png)
.png)